The Tula pivotal study
A prospective, multicenter study evaluating the safety and effectiveness of in-office ear tube placement with the Tula System in children aged 6 months to 12 years. 337 Children were enrolled into one of three successive cohorts: Operating room lead-in (n=68), Office lead-in (n=47), Pivotal (n=222, in-office).
Safe
Zero serious device, drug, or procedure-related adverse events1
There were zero serious device, drug, or procedure-related adverse events in the study. Patients were treated in-office without use of sedation, anxiolytics or mechanical restraints. Behavior management techniques of distraction (toys, videos), engagement with patient, and framing (age-appropriate communication about the procedure) were among the methods used to minimize patient anxiety and distress.1
Practical
overall in-office success rate1
Procedural success was defined as tubes placed successfully in both ears for a bilateral case. 91% of patients in the Tula pivotal study were bilaterally indicated. Unsuccessful procedures were most commonly due to behavior (5%) or inadequate anesthesia (3.2%). In more than half (53.6%) of the cases that failed bilateral tube placements, doctors were able to successfully insert an ear tube in one ear.2
High parent satisfaction
of parents were very satisfied with the Tula procedure1
Parent satisfaction was recorded at the 3-week follow-up with 95% percent of parents noting “Strongly Agree” (82%) or “Agree” (13%) to the following statement: “Overall, I am very satisfied with the in-office ear tube procedure.” A total of 201 parents completed the survey.
In-office tympanostomy tube placement in children using iontophoresis and automated tube delivery.
Lustig LR, et al. Laryngoscope (2020) Read Now
Initial publication of the procedural and post-procedural results of the Tula pivotal study through 6-months follow up. Provides additional analyses and discussion on retention, patency, and effusion resolution.
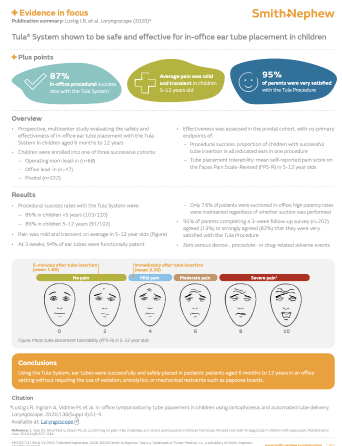
- High patency despite low suction rates1
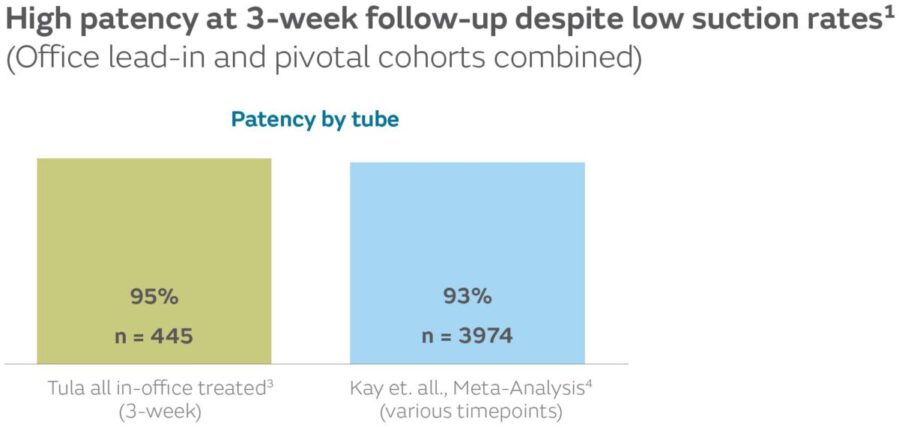
- Effusion clears without suction1
95%
At 3-week follow-up, 95% of tubes were functionally patent for the Office lead-in and Pivotal cohorts combined (n=445). Suction was performed in 12.3% and 7.6% of Office lead-in and Pivotal patients, respectively. High patency rates were maintained regardless of whether suction was performed.1
96.5%
57.9% of total ears had effusion at the time of the procedure. 96.5% of ears with mucoid effusion at the time of surgery were clear at 3-week follow-up.1,5
Two-Year Outcomes After Pediatric In-Office Tympanostomy Using Lidocaine/Epinephrine Iontophoresis and an Automated Tube Delivery System
Waldman EH, et al. Otolaryngol Head Neck Surg (2023) Read Now
Extends the findings of the Tula pivotal study to show successful tube placement was accompanied by positive long‐term outcomes for tube retention, patency, and safety. Patients were followed for 2 years or until tube extrusion, whichever occurred first.
- Mean intubation period
- Tube retention
16.8Months
In the Tula pivotal study, the mean intubation period was 16.8 months (median 15.8 months) for all cohorts combined, consistent with ranges reported in the literature for similar tubes placed in the OR (7-18.5 months). Probable date of extrusion was calculated as the midpoint between the date when tube position across the tympanic membrane was last confirmed and the date when the tube was first noted to be extruded.6-14
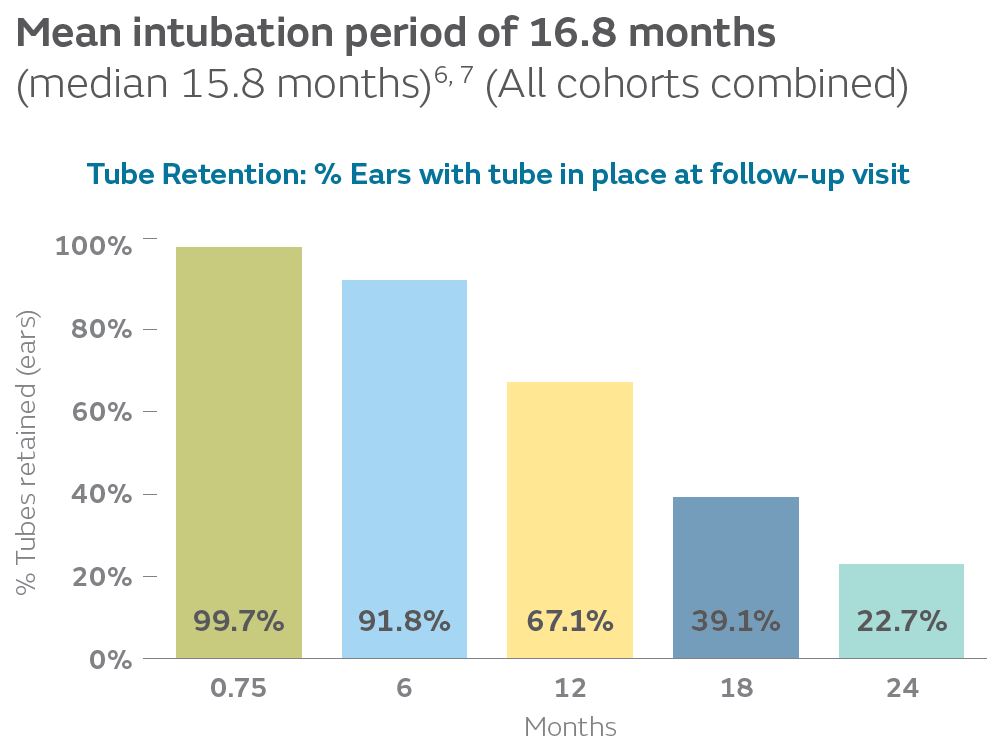
23% retention rate at 2-years
In the Tula pivotal study, 23% of ears had Tula tubes remaining in place at 24-month follow-up, consistent with the literature for common tympanostomy tubes (0-32%).6, 8, 9, 15, 16

Related papers
Contact Us
To learn more about the Tula System, ask a question, or speak with your local Sales Representative, send us a message.